Questo sito purtroppo è stato chiuso.
Scuola Eco Color Doppler e mezzo di contrasto
 La “Scuola di formazione ecografia specialistica di color Doppler e mezzo di contrasto” segue il regolamento generale delle scuole S.I.U.M.B per l'addestramento teorico pratico in ecografia. L’iscrizione alla scuola è riservata a tutti i laureati in Medicina e Chirurgia, iscritti all’ordine dei Medici >>>
La “Scuola di formazione ecografia specialistica di color Doppler e mezzo di contrasto” segue il regolamento generale delle scuole S.I.U.M.B per l'addestramento teorico pratico in ecografia. L’iscrizione alla scuola è riservata a tutti i laureati in Medicina e Chirurgia, iscritti all’ordine dei Medici >>>
Corso di Imaging Clinico 2016 a Ghilarza
(Venerdì 9 Dicembre 2016)
(Venerdì 9 Dicembre 2016)
 L’imaging Clinico rappresenta in Sardegna da anni un importante occasione di aggiornamento culturale per gli ecografisti e per tutti i cultori dell’imaging. L’incontro del 2016 ha lo scopo di illustrare... >>>
L’imaging Clinico rappresenta in Sardegna da anni un importante occasione di aggiornamento culturale per gli ecografisti e per tutti i cultori dell’imaging. L’incontro del 2016 ha lo scopo di illustrare... >>>
Last articles published
Contrast-Enhanced UltraSonography of pa...
Lectures
2010-05-08

Focal liver lesion: nonlinear contrast-...
Lectures
2008-11-15
Musculoskeletal ultrasound technical gu...
Lectures
2007-11-16

Leydig cell tumor US finding in older male
Clinical cases
2006-02-03

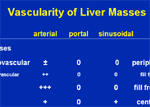
Role of image-guided therapies in hepat...
Lectures
2006-01-26
Contrast Harmonic Imaging
Studies
2005-03-02
Contrast media imaging: introduction to...
Tecnological advances
2004-04-30
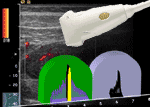
Small parts and contrast media: problem...
Contrast media
2004-03-30
Ultrasound of the Wrist and Hand (Marti...
Lectures
2003-11-14
Harmonic Imaging: tissues and microbubb...
Lectures
2003-07-08
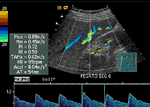
Abnormal Intrahepatic Vascular Shunt
Educational
2003-05-08
Tissue Harmonic Imaging
Studies
2003-04-02
News
Corso di Imaging Clinico 2016 a Ghilarza 2016-11-11
Pubblicata nella sezione lezioni la pagina sul "Corso di Imaging Clinico 2016" che si terrà il 9 dicembre 2016 presso Sas Mendulas a Ghilarza (OR).
Corso di Imaging Clinico 2015 ad Abbasanta 2015-11-24
Pubblicata nella sezione lezioni la pagina sul "Corso di Imaging Clinico 2015" che si terrà il 4 dicembre 2015 presso l'Hotel Su Baione ad Abbasanta.
Corso di Imaging Clinico 2014 a Nuoro 2014-12-08
Pubblicata nella sezione lezioni la pagina sul "Corso di Imaging Clinico 2014" che si terrà il 19 dicembre 2014 presso AILUN - SIMANNU Centro di Simulazione Medica (Nuoro).
Corso di Imaging Clinico 2013 a Borutta 2013-11-14
Pubblicata nella sezione lezioni la pagina sul "Corso di Imaging Clinico 2013 " che si terrà il 14 dicembre 2013 presso la Foresteria del Monastero di S. Pietro di Sorres (Borutta)
Aggiornamenti nell’Imaging Clinico 2012 ad Alghero 2012-04-22
Pubblicata nella sezione lezioni la pagina sul congresso "Aggiornamenti nell’Imaging Clinico 2012. Corso color doppler e ecocontrastografia: dalla valutazione del flusso alla valutazione della perfusione" che si terrà il 15 maggio 2012 presso Villa Loreto ad Alghero (SS)
Aggiornamenti nell’Imaging Clinico 2011 ad Alghero 2011-03-08
Pubblicata nella sezione lezioni la pagina sul congresso "Aggiornamenti nell’Imaging Clinico 2011: corso di formazione, dal color doppler alla contrastografia" che si terrà il l'8 aprile 2011 presso l'Hotel Catalunya ad Alghero
Corso pratico per lo studio ecografico del sistema nervoso periferico a Sassari 2010-09-05
Pubblicato il programma del "Corso pratico per lo studio ecografico del sistema nervoso periferico" che si terrà il 5 ottobre 2010 a Sassari.
Aggiornamenti nell’Imaging Clinico 2010 a Macomer 2010-08-31
Pubblicata nella sezione lezioni la pagina sul congresso "Aggiornamenti nell’Imaging Clinico 2010: ruolo dell’eco-contrastografia, nuove linee guida e ricadute farmaco economiche nuovi orizzonti nelle terapie loco regionali eco-guidate" che si terrà il 9 e 10 settembre 2010 presso il centro congressi di Filigosa (ex Caserme Mura) a Macomer (NU)
SMIRG at RSNA 2009 2009-11-13
Abstract of oral presentations by SMIRG at the congress of Radiological Society of North America (RSNA 2009) are published in research section.
Attualità nell’Imaging Clinico a Macomer 2009-04-03
Pubblicata nella sezione lezioni la pagina sul congresso "Attualità nell’Imaging Clinico: ruolo dell’eco-contrastografia, nuove linee guida e ricadute farmaco economiche nuovi orizzonti nelle terapie loco regionali eco-guidate" che si terrà il 14 e 15 maggio 2009 presso il centro congressi di Filigosa (ex Caserme Mura) a Macomer (NU)
Diagnostica e procedure interventistiche in senologia a Cagliari 2008-09-20
Pubblicata nella sezione lezioni la pagina sul congresso "Diagnostica e procedure interventistiche in senologia: lo stato dell’arte" che si terrà il 10 ottobre 2008 presso il THotel a Cagliari
Corsi Martedì Ecografico 2008 2008-04-13
Pubblicato nella sezione Lezioni e attività il programma del corso "Aggiornamenti in tema di ecografia: Patologie autoimmuni e degenerative" che si terrà a Cagliari il 13 maggio 2008.
Corsi Martedì Ecografico 2008 2007-12-22
Pubblicato nella sezione Lezioni e attività il programma del corso "Aggiornamenti in tema di ecografia: Patologie tumorali" che si terrà a Cagliari il 22 gennaio 2008.
SMIRG at RSNA 2007 2007-11-23
Abstract of oral presentations by SMIRG at the congress of Radiological Society of North America (RSNA 2007) are published in research section.
Diagnostica per immagini a Su Gologone 2007-04-23
Pubblicata nella sezione lezioni la pagina sul congresso "Diagnostica per immagini a Su Gologone - Attualità nell’Imaging Clinico e Terapeutico" che si terrà il 25 e 26 maggio 2007 presso l'hotel Su Gologone a Oliena (NU)
SMIRG at RSNA 2006 2006-10-06
Abstract of oral presentation "Modern Ultrasound Evaluation of Inflammatory Activity in Crohn's Disease" by SMIRG at the congress of Radiological Society of North America (RSNA) is Published in research section.
Mediterranean Meeting of Clinical Imaging 2006-02-11
Sono aperte le iscrizioni al congresso "Incontro Mediterraneo di Imaging Clinico" che si terrà a Orosei (Nùgoro, Sardinia - I) in data 11 marzo 2006. Il modulo di iscrizione e le relative istruzioni sono disponibili nella sezione "Lezioni e attività".
Corsi Martedì Ecografico 2004-11-25
Pubblicato nella sezione Lezioni e attività il calendario dei "Corsi Martedì Ecografico" che si terranno a Cagliari da marzo a giugno 2005.
